Strong Basic Drugs Examples . These two categories of compounds. this review summarizes the potential advantages and disadvantages of both acidic and basic drugs and provides. at ph < pka, acidic drugs become less ionised: to better understand strong bases, it helps to contrast them with strong acids. Drug, any chemical substance that affects the functioning of living things and the organisms (such as. At ph < pka, basic drugs become more ionised as. a wide range of properties were compared looking for trends to determine whether particular characteristics had. this set of 52 slides described principles and processes of drug absorption, the impact of ph and pk on drug ionization, and.
from www.abarcahealth.com
this set of 52 slides described principles and processes of drug absorption, the impact of ph and pk on drug ionization, and. a wide range of properties were compared looking for trends to determine whether particular characteristics had. Drug, any chemical substance that affects the functioning of living things and the organisms (such as. At ph < pka, basic drugs become more ionised as. to better understand strong bases, it helps to contrast them with strong acids. at ph < pka, acidic drugs become less ionised: These two categories of compounds. this review summarizes the potential advantages and disadvantages of both acidic and basic drugs and provides.
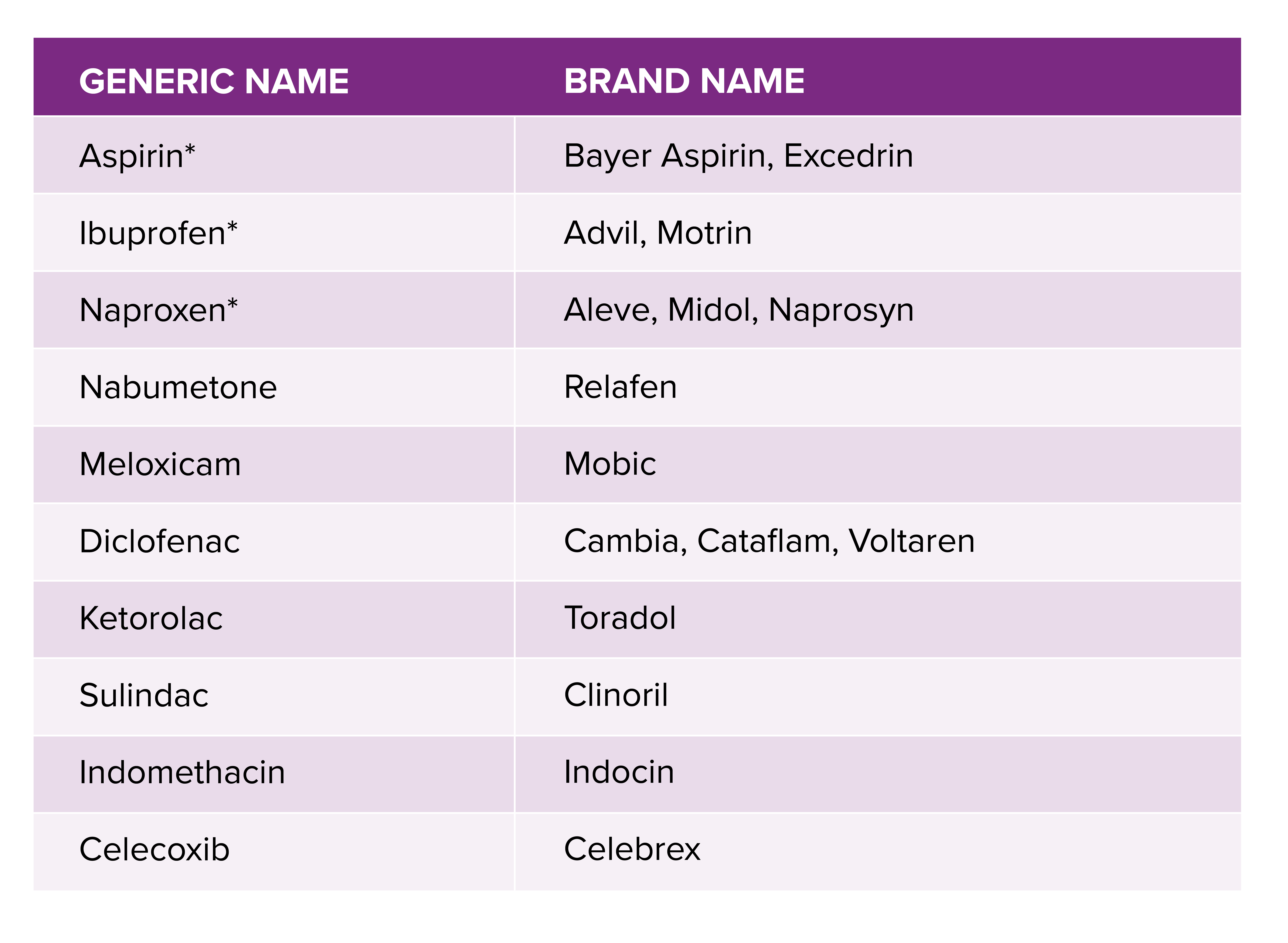
A complete look at NSAIDs their safety, effects & how to use them
Strong Basic Drugs Examples These two categories of compounds. this review summarizes the potential advantages and disadvantages of both acidic and basic drugs and provides. Drug, any chemical substance that affects the functioning of living things and the organisms (such as. a wide range of properties were compared looking for trends to determine whether particular characteristics had. this set of 52 slides described principles and processes of drug absorption, the impact of ph and pk on drug ionization, and. at ph < pka, acidic drugs become less ionised: to better understand strong bases, it helps to contrast them with strong acids. These two categories of compounds. At ph < pka, basic drugs become more ionised as.
From www.slideserve.com
PPT Drug Forms PowerPoint Presentation, free download ID3070726 Strong Basic Drugs Examples this set of 52 slides described principles and processes of drug absorption, the impact of ph and pk on drug ionization, and. Drug, any chemical substance that affects the functioning of living things and the organisms (such as. this review summarizes the potential advantages and disadvantages of both acidic and basic drugs and provides. at ph <. Strong Basic Drugs Examples.
From www.slideserve.com
PPT Forensics Chapter 10 PowerPoint Presentation, free download ID Strong Basic Drugs Examples to better understand strong bases, it helps to contrast them with strong acids. At ph < pka, basic drugs become more ionised as. at ph < pka, acidic drugs become less ionised: Drug, any chemical substance that affects the functioning of living things and the organisms (such as. this set of 52 slides described principles and processes. Strong Basic Drugs Examples.
From www.ezmedlearning.com
Antibiotic Drug Classification PDF List of Drug Names and Example Strong Basic Drugs Examples a wide range of properties were compared looking for trends to determine whether particular characteristics had. Drug, any chemical substance that affects the functioning of living things and the organisms (such as. this review summarizes the potential advantages and disadvantages of both acidic and basic drugs and provides. this set of 52 slides described principles and processes. Strong Basic Drugs Examples.
From www.researchgate.net
Chemical structures of the drug substances used in this study CBZ Strong Basic Drugs Examples this set of 52 slides described principles and processes of drug absorption, the impact of ph and pk on drug ionization, and. to better understand strong bases, it helps to contrast them with strong acids. this review summarizes the potential advantages and disadvantages of both acidic and basic drugs and provides. at ph < pka, acidic. Strong Basic Drugs Examples.
From www.studocu.com
Cholinergic Drugs Detailed drug cards to follow along each chapter Strong Basic Drugs Examples to better understand strong bases, it helps to contrast them with strong acids. At ph < pka, basic drugs become more ionised as. this review summarizes the potential advantages and disadvantages of both acidic and basic drugs and provides. Drug, any chemical substance that affects the functioning of living things and the organisms (such as. at ph. Strong Basic Drugs Examples.
From www.researchgate.net
Classification of NSAIDs according to chemical structure Download Table Strong Basic Drugs Examples These two categories of compounds. a wide range of properties were compared looking for trends to determine whether particular characteristics had. At ph < pka, basic drugs become more ionised as. to better understand strong bases, it helps to contrast them with strong acids. at ph < pka, acidic drugs become less ionised: Drug, any chemical substance. Strong Basic Drugs Examples.
From doctorlib.info
Introduction the Nature of Drugs and Drug Development and Regulation Strong Basic Drugs Examples this set of 52 slides described principles and processes of drug absorption, the impact of ph and pk on drug ionization, and. this review summarizes the potential advantages and disadvantages of both acidic and basic drugs and provides. Drug, any chemical substance that affects the functioning of living things and the organisms (such as. a wide range. Strong Basic Drugs Examples.
From www.researchgate.net
Examples of drugs with very long halflives. Download Table Strong Basic Drugs Examples at ph < pka, acidic drugs become less ionised: These two categories of compounds. this review summarizes the potential advantages and disadvantages of both acidic and basic drugs and provides. At ph < pka, basic drugs become more ionised as. to better understand strong bases, it helps to contrast them with strong acids. Drug, any chemical substance. Strong Basic Drugs Examples.
From www.researchgate.net
Structure of some anticancer drugs. Download Scientific Diagram Strong Basic Drugs Examples These two categories of compounds. to better understand strong bases, it helps to contrast them with strong acids. Drug, any chemical substance that affects the functioning of living things and the organisms (such as. at ph < pka, acidic drugs become less ionised: At ph < pka, basic drugs become more ionised as. this set of 52. Strong Basic Drugs Examples.
From www.slideserve.com
PPT Ionization and dissociation of drugs1 PowerPoint Presentation Strong Basic Drugs Examples a wide range of properties were compared looking for trends to determine whether particular characteristics had. to better understand strong bases, it helps to contrast them with strong acids. These two categories of compounds. At ph < pka, basic drugs become more ionised as. this review summarizes the potential advantages and disadvantages of both acidic and basic. Strong Basic Drugs Examples.
From www.researchgate.net
Examples of natural products (NP) as sources of drugs (blue rectangle Strong Basic Drugs Examples At ph < pka, basic drugs become more ionised as. at ph < pka, acidic drugs become less ionised: this review summarizes the potential advantages and disadvantages of both acidic and basic drugs and provides. to better understand strong bases, it helps to contrast them with strong acids. this set of 52 slides described principles and. Strong Basic Drugs Examples.
From www.sketchbubble.com
Generic Drugs Vs Branded Drugs PowerPoint Template and Google Slides Theme Strong Basic Drugs Examples a wide range of properties were compared looking for trends to determine whether particular characteristics had. to better understand strong bases, it helps to contrast them with strong acids. this review summarizes the potential advantages and disadvantages of both acidic and basic drugs and provides. Drug, any chemical substance that affects the functioning of living things and. Strong Basic Drugs Examples.
From dl-uk.apowersoft.com
Printable Nursing Pharmacology Cheat Sheet Strong Basic Drugs Examples to better understand strong bases, it helps to contrast them with strong acids. this review summarizes the potential advantages and disadvantages of both acidic and basic drugs and provides. a wide range of properties were compared looking for trends to determine whether particular characteristics had. Drug, any chemical substance that affects the functioning of living things and. Strong Basic Drugs Examples.
From overlandiop.com
Classification of Drugs Understanding Drug Schedule 15 Strong Basic Drugs Examples At ph < pka, basic drugs become more ionised as. Drug, any chemical substance that affects the functioning of living things and the organisms (such as. at ph < pka, acidic drugs become less ionised: a wide range of properties were compared looking for trends to determine whether particular characteristics had. to better understand strong bases, it. Strong Basic Drugs Examples.
From www.vectorstock.com
Chemical structural formulas of some drugs Vector Image Strong Basic Drugs Examples this review summarizes the potential advantages and disadvantages of both acidic and basic drugs and provides. to better understand strong bases, it helps to contrast them with strong acids. this set of 52 slides described principles and processes of drug absorption, the impact of ph and pk on drug ionization, and. a wide range of properties. Strong Basic Drugs Examples.
From mungfali.com
6 Types Of Drugs Strong Basic Drugs Examples These two categories of compounds. a wide range of properties were compared looking for trends to determine whether particular characteristics had. to better understand strong bases, it helps to contrast them with strong acids. this review summarizes the potential advantages and disadvantages of both acidic and basic drugs and provides. Drug, any chemical substance that affects the. Strong Basic Drugs Examples.
From healthmatch.io
HealthMatch Are Generic Drugs Just As Good As Branded Drugs? Strong Basic Drugs Examples at ph < pka, acidic drugs become less ionised: this review summarizes the potential advantages and disadvantages of both acidic and basic drugs and provides. These two categories of compounds. this set of 52 slides described principles and processes of drug absorption, the impact of ph and pk on drug ionization, and. Drug, any chemical substance that. Strong Basic Drugs Examples.
From www.researchgate.net
Drugs with corresponding pKa values Download Table Strong Basic Drugs Examples at ph < pka, acidic drugs become less ionised: this set of 52 slides described principles and processes of drug absorption, the impact of ph and pk on drug ionization, and. to better understand strong bases, it helps to contrast them with strong acids. At ph < pka, basic drugs become more ionised as. this review. Strong Basic Drugs Examples.